The periodic table of elements is the cornerstone of chemistry, providing a systematic arrangement of elements based on their atomic number, electron configuration, and recurring chemical properties. Among these elements is lead, represented by the symbol "Pb" from the Latin word "plumbum." This heavy metal has a rich history and plays a significant role in various industries today. Understanding the Pb periodic table not only sheds light on lead's properties but also its applications and implications for health and the environment.
Lead has been utilized by humans for thousands of years, dating back to ancient civilizations. Its malleability, density, and resistance to corrosion made it a popular choice for a variety of uses, including plumbing, roofing, and even cosmetics. However, as knowledge about the toxicity of lead has increased, its use has come under scrutiny, leading to regulations and a decline in many of its traditional applications. This evolution highlights the importance of the Pb periodic table in understanding not just the element itself, but also the broader context of chemistry and public health.
As we dive deeper into the Pb periodic table, we will explore the characteristics of lead, its historical significance, and its impact on modern society. This journey will not only enhance our appreciation for this element but also inform us about the ongoing challenges and innovations related to lead in various industries.
What is Lead (Pb) and Why is it Important?
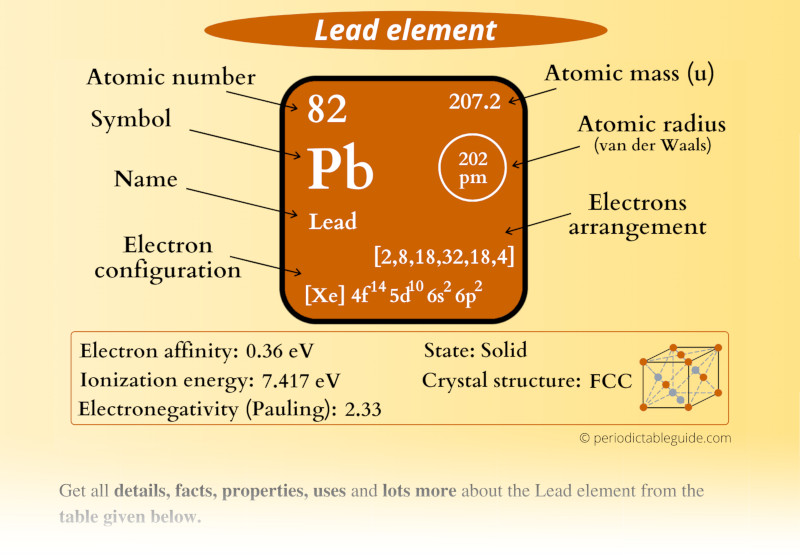
Lead, with the atomic number 82, is classified as a heavy metal and belongs to group 14 of the periodic table. It is known for its high density and low melting point. Lead is often found in nature in combination with other elements, primarily in ores such as galena. Its unique properties have made it a valuable resource throughout history.
What are the Physical and Chemical Properties of Pb?
The properties of lead are essential to understanding its behavior in various applications. Here are some key physical and chemical properties:
- Atomic Number: 82
- Atomic Weight: 207.2 g/mol
- Melting Point: 327.5 °C (621.5 °F)
- Boiling Point: 1749 °C (3180 °F)
- Density: 11.34 g/cm³
- Electron Configuration: [Xe] 4f14 5d10 6s2 6p2
How is Lead Obtained and Processed?
Lead is primarily extracted from lead sulfide ore, commonly known as galena. The extraction process involves crushing the ore and then using various methods, such as flotation or smelting, to separate lead from other materials. Once extracted, lead is refined to remove impurities, resulting in pure lead that can be utilized in various industrial applications.
What are the Common Uses of Lead?
Lead has numerous applications across different sectors. Here are some of the most common uses:
- Batteries: Lead-acid batteries are widely used in vehicles and backup power systems.
- Radiation Shielding: Due to its dense nature, lead is used to shield against radiation in medical and industrial settings.
- Construction: Lead is used in roofing materials, pipes, and solder.
- Paints and Pigments: Although its use has decreased due to health concerns, lead was historically used in paints for its durability and color.
What are the Health Risks Associated with Lead Exposure?
Despite its various applications, lead poses significant health risks, especially concerning lead exposure. Prolonged exposure to lead can lead to serious health issues, particularly in children. Some of the health effects include:
- Neurological damage
- Developmental delays in children
- Kidney damage
- High blood pressure
- Anemia
How is Lead Regulated and Monitored?
Given the dangers associated with lead, many countries have established regulations to limit its use and exposure. In the United States, the Environmental Protection Agency (EPA) and the Occupational Safety and Health Administration (OSHA) enforce standards to protect public health. Monitoring programs are also in place to assess lead levels in the environment and in human populations.
What is the Future of Lead in Industry?
The future of lead in industry is likely to involve continued innovation and regulation. As technology advances, new materials are being developed to replace lead in various applications, particularly in electronics and construction. However, lead will likely remain an important material in specific sectors, such as battery manufacturing and radiation protection, as long as its use is managed responsibly.
How Can We Safely Handle Lead?
For those working with lead or living in environments with potential lead exposure, safety precautions are essential. Here are some tips for safe handling:
- Use personal protective equipment (PPE) such as gloves and masks.
- Ensure proper ventilation when working with lead materials.
- Regularly monitor lead levels in the workplace or home.
- Follow local regulations and guidelines for lead handling and disposal.
Conclusion: The Pb Periodic Table and Its Significance
In conclusion, the Pb periodic table represents more than just a chemical element; it is a symbol of the intersection between human innovation and health awareness. While lead has historically played a crucial role in various industries, its health risks cannot be overlooked. Understanding the properties and implications of lead is vital for ensuring its safe use and managing its impact on public health and the environment.
As we continue to uncover the complexities of the Pb periodic table, it is our responsibility to educate ourselves and others about the safe handling and use of lead, ensuring that we can harness its benefits while minimizing its risks.
Article Recommendations
- Angie Dickenson An Iconic Actress And Her Impact On Hollywood
- All About Bloodhound Lil Jeff A Rising Stars Journey
- Mitch Mcconnell Snopes Debunked Facts Amp Rumors